Background:
The mainstay treatment for mantle cell lymphoma (MCL) is chemotherapy ± immunotherapy. The standard chemotherapeutic regimens have limited efficacy in MCL when used alone. In this systematic review, we have assessed the efficacy and safety of various combination regimens for the treatment of MCL evaluated in phase III clinical trials.
Methods:
We performed a comprehensive systematic literature search on PubMed, Embase, clinicaltrials.gov, and Web of Science databases with the date of inception to May 2020. We used MeSH (Medical Subject Headings) terms for "mantle cell lymphoma", "treatment outcome" along with their keywords, and combined their results. Our search generated a total of 3572 articles. After excluding case reports, case series, observational studies, review articles, meta-analysis, phase I/II clinical trials, and pre-clinical studies, we included five phase III randomized clinical trials (RCTs) reporting the efficacy of combination regimens for MCL treatment.
Results
Data from five phase III RCTs was pooled with total N=1683 (newly diagnosed (ND), n=1242, relapsed/refractory (RR), n=441). 1610 patients were evaluable. Kluin-Nelemans et al. 2020 (n=560) studied induction with R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) vs R-FC (rituximab, fludarabine, and cyclophosphamide) for ND-MCL with follow-up on maintenance with rituximab (R) -vs interferon alfa. At a median follow up of 7.6 years (y), median overall survival (mOS) was 6.4 vs 3.9 y (p=0.0054) in R-CHOP vs R-FC group, respectively. The median progression-free survival (mPFS) and mOS in the R-CHOP cohort on R maintenance were significantly better when compared to those on interferon alfa maintenance (mPFS, 5.4 y vs 1.9 y, p<0.001) (mOS, 9.8 y vs 7.1 y, p=0.0026).
Robak et al. (2015) (n=487) assessed bortezomib in ND-MCL by substituting it for vincristine in the standard R-CHOP therapy. A cohort of MCL ineligible for stem cell transplant (SCT) was randomly assigned to either R-CHOP or VR-CAP (bortezomib replacing vincristine). At a median follow-up of 40 months (mo), mPFS was 24.7 mo vs 14.4 mo in VR-CAP vs R-CHOP (HR 0.63, p<0.001), respectively. Similar, relative improvements were reported in complete response (CR) (53% vs 42%, p=0.007) and 4-year OS was (64% [95% CI 56-71] vs 54% [95% CI 45-62]).
Jin et al. (2018) (n=121) assessed R-CHOP vs VR-CAP in ND-MCL patients ineligible for SCT. After a median follow-up of 42.4 mo, mPFS for VR-CAP was better than R-CHOP (28.6 mo vs 13.9 mo, HR=0.7, p=0.157). The overall response rate (ORR) was almost similar in VR-CAP (97%) and R-CHOP (98%). The 4-year OS was 62% vs 61% in VR-CAP vs R-CHOP, respectively.
Flinn et al. (2014) (n=74) studied the efficacy of bendamustine in combination with rituximab (BR) vs R-CHOP or R-CVP (R-CHOP minus doxorubicin) as induction regimens in ND-MCL. 36 patients received BR and 38 patients received R-CHOP/R-CVP. ORR was 94% vs 85% with BR vs R-CHOP/R-CVP, respectively.
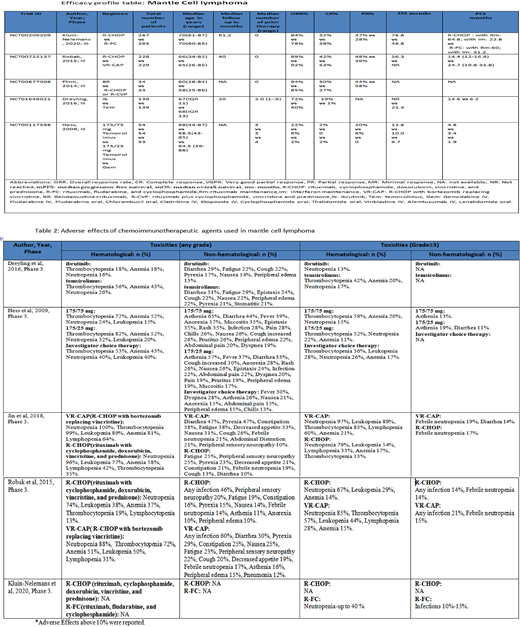
Hess et al. (2009) (n=162) evaluated temsirolimus in RR-MCL. The study population was randomized to either 175/75mg temsirolimus (arm A), 175/25mg temsirolimus (arm B), and the investigator's choice of chemotherapy (arm C). The mPFS was significantly better in arm A compared to arm C (4.8 mo vs 1.9 mo, HR 0.44 [97.5% CI 0.25-0.7], p=0.0009). Arm B had slight improvement but insignificant. Similarly, mOS was 12.8 mo in arm A (HR 0.8 [95% CI 0.5-1.28], p=0.35), and 8.8 in arm B (HR 0.96 [95% CI 0.60-1.54], p=0.87), when compared to 9.5 mo in arm C. Drelying et al. (2016) (n=280), studied temsirolimus in comparison with ibrutinib, RR-MCL with mPFS of 14.6 mo (95% CI 10.4-not estimable) vs 6.2 mo (95% CI 4.2-7.9), respectively.
Toxicities were typically manageable. VR-CAP was associated with a higher incidence of toxicity (100%) vs R-CHOP (94%) majority of which was hematological. Thrombocytopenia was particularly more prominent with temsirolimus. Granulocytopenia was persistent in 30-40% in the R-FC cohort after 5 y.
Conclusion:
The chemo-immunotherapy combination is favorable compared to chemotherapy alone for the treatment of MCL. R-CHOP induction followed by rituximab maintenance in MCL shows favorable long-term safety and efficacy profile. Bortezomib substituting for vincristine in R-CHOP improves the efficacy outcomes. Ibrutinib-based regimens are superior to temsirolimus-based regimens.
Anwer:Incyte, Seattle Genetics, Acetylon Pharmaceuticals, AbbVie Pharma, Astellas Pharma, Celegene, Millennium Pharmaceuticals.:Honoraria, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.